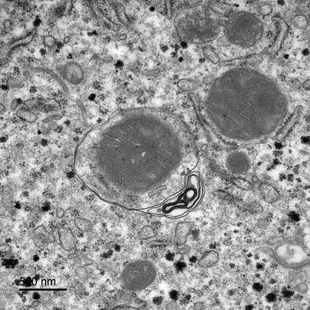
Autophagy is a crucial metabolic regulator. In a recent study, researchers investigated in-depth how liver autophagy is altered by food deprivation. The AgRP neurons in the hypothalamus, activated by energy deficiency, play a crucial role.
The hypothalamus is essential for energy homeostasis regulation. Special neurons receive hormonal, neuronal, and nutritional physiological signals to fulfill this role. These include AgRP neurons (Agouti-Related Peptide), which recognize and integrate metabolic signals. This helps regulate food ingestion and energy expenditure and maintain glucose homeostasis.
AgRP Neuron-Induced Hepatic Autophagy
A team led by the DZD-associated partner Prof. Jens Brüning from the Max Planck Institute for Metabolism Research in Cologne discovered that fasting in mice activated the AgRP neurons in their hypothalamus. In turn, this induced autophagy in the liver, promoting ketogenesis.
Autophagy is an autonomous cellular process during which digestive components, such as proteins, lipids, and nucleic acids, are transported to the lysosomes. This maintains cellular homeostasis and provides metabolic substrates for energy production during fasting. Further experiments found that AgRP neuron-induced hepatic autophagy depends on neuropeptide Y (NPY) release from the paraventricular nucleus of the hypothalamus (PVH) and activates PVHCRH neurons.
“The activation of the AgRP neurons increased the concentration of circulating corticosteroids,” explains DZD researcher Prof. Dr. Jens Claus Brüning. Hepatic glucocorticoid receptor expression reduction weakened the AgRP neuron-dependent activation of hepatic autophagy. Hepatic autophagy ceased after the researchers inhibited the AgRP neurons during energy deprivation.
Adapting to a Negative Energy Status
The findings indicated the following mechanism: During a short fasting period, AgRP neurons release NPY, promoting a neuronal circuit for PVHCRH activation. This, in turn, activates the hypothalamic-pituitary-adrenal axis and glucocorticoid release, which controls hepatic autophagy.
This enables adaptation to negative energy status. “The results contribute to a better understanding of the regulatory principle of liver autophagy in controlling metabolic adaptation during food deprivation.” says Brüning.
Original publication:
Weiyi Chen, Oliver Mehlkop , Alexandra Scharn, Hendrik Nolte, Paul Klemm, Sinika Henschke, Lukas Steuernagel, Tamara Sotelo-Hitschfeld, Ecem Kaya, Claudia Maria Wunderlich, Thomas Langer, Natalia L Kononenko, Patrick Giavalisco & Jens Claus Brüning. Nutrient-sensing AgRP neurons relay control of liver autophagy during energy deprivation. Cell Metab. 2023 May. doi: 10.1016/j.cmet.2023.03.019.