Dresden, 15.11.2019
Researchers discover a new way in which insulin interacts with its receptor
Since the discovery of insulin nearly 100 years ago, scientists have explored how it interacts with its receptor with the goal of improving the ability of therapeutic insulins to mimic the way insulin works in the body. In a new study, researchers have solved a critical piece of the puzzle by showing how insulin interacts with its receptor at a second binding site. The team of scientists from the Paul Langerhans Institute Dresden (a satellite of Helmholtz Zentrum München and a partner of the German Center for Diabetes Research) and the Faculty of Medicine Carl Gustav Carus at TU Dresden in Germany, together with colleagues from the Max Planck Institute of Biochemistry in Munich, Germany, McGill University in Canada, and the University of Helsinki in Finland will publish their results in the Journal of Cell Biology (JCB).
Previous studies have detailed the central role of insulin as a regulator of blood sugar and demonstrated its involvement in diabetes and other chronic conditions, including neurodegeneration and cancer. The biological actions of insulin are mediated by its receptor—the insulin receptor—which is localized on the cell surface.
“When insulin was administered to patients for the first time in the 1920s, it was a real breakthrough in the treatment of diabetes. However, it is still challenging to generate insulins that recapitulate the full spectrum of endogenous insulin action,” explains Dr. Ünal Coskun, group leader at the Institute for Pancreatic Islet Research (IPI) and Paul Langerhans Institute Dresden (PLID), a partner of the German Center for Diabetes Research (DZD). “The main reason for this is that we still do not understand enough about how insulin binds to its receptor and how this signal is transmitted inside the cell.”
Insulin was first proposed to bind to two different sites on its receptor 40 years ago. Although much is understood about the interactions that occur at the first of these sites, very little was known about what happens at second site. Understanding the different ways that insulin can interact with its receptor will allow researchers to design improved treatments for insulin-related disorders.
In the new study, the researchers show how insulin binds to site 2. Using a powerful technique known as cryo-electron microscopy, the researchers obtain a detailed 3D picture of the insulin receptor ectodomain bound to insulin.
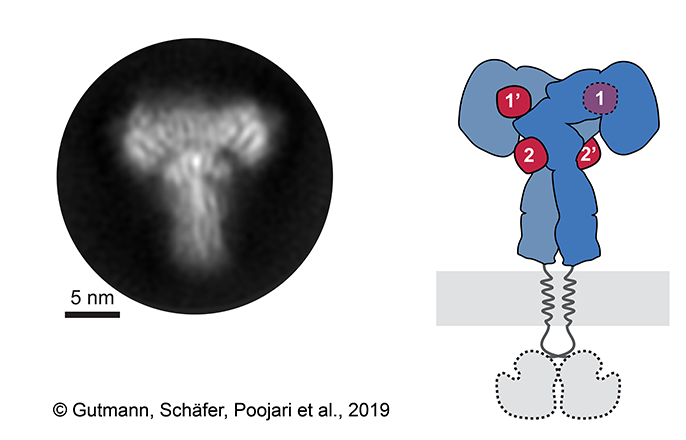
“The key was to examine the outer part, or ectodomain, of the insulin receptor after saturating it with high concentrations of insulin,” explains Dr. Theresia Gutmann, co-first author of the study from the Paul Langerhans Institute and the German Center for Diabetes Research. Co-first author Dr. Ingmar Schäfer, from the Department of Structural Cell Biology at the Max-Planck Institute of Biochemistry (MPIB) adds, “We recorded over 8,000 electron microscopic images and analyzed over 300,000 single receptor particles, from which we could generate 2D images of the “T”-shaped complex to reconstruct a 3D image.”
This approach allowed the researchers to directly observe the binding of insulin to site 2 for the first time and to show how the insulin receptor changes its conformation to form a T-shaped structure. The receptor consists of two identical parts, each containing two insulin binding sites, so up to 4 insulin molecules were bound by a single receptor (Figure 1).
In parallel, the scientists used computational modelling and simulation approaches to understand these interactions on an atomic scale. “Computational techniques such as these are becoming increasingly important to analyze complicated dynamic processes in living cells, with the added advantage of allowing drug screens to be performed in silico,” says Prof. Ilpo Vattulainen from the Department of Physics at the University of Helsinki.
Previous studies analyzing the structure of the insulin receptor were difficult to reconcile with biochemical and genetic data on how insulin interacts with its receptor. The scientists hope that these new details concerning insulin–receptor interactions will ultimately expand the current models of insulin binding to its receptor and pave the way towards new approaches to structure-based drug design.

Figure 1: The insulin receptor saturated with insulin molecules. A representative 2D class average of the insulin receptor ectodomain saturated with insulin obtained by cryo-electron microscopy (left) and the corresponding scheme of the complete receptor (right). The insulin receptor ectodomain and the four insulins are colored in blue and red, respectively. See also in a video
Further Information
Original Publication:
Gutmann, Schäfer, Poojari, Brankatschk, Vattulainen, Strauss, and Coskun. (2019): Cryo-EM structure of the complete and ligand-saturated insulin receptor ectodomain. Journal of Cell Biology, DOI: 10.1083/jcb.201907210
The Paul Langerhans Institute of the Helmholtz Zentrum München at the University Hospital Carl Gustav Carus and the Medical Faculty of TU Dresden (PLID) contributes decisively to a better understanding of the mechanisms of the disease and to explore new therapeutic options. The institute is a founding-partner of the German Center for Diabetes Research (DZD e.V.) and has been a satellite institute of the Helmholtz Zentrum München since January 2015. Working in the DZD network allows research projects of a much larger scale, both in the area of basic research through interdisciplinary approaches as well as in the area of clinical studies.
The German Center for Diabetes Research (DZD) is a national association that brings together experts in the field of diabetes research and combines basic research, translational research, epidemiology and clinical applications. The aim is to develop novel strategies for personalized prevention and treatment of diabetes. Members are Helmholtz Zentrum München – German Research Center for Environmental Health, the German Diabetes Center in Düsseldorf, the German Institute of Human Nutrition in Potsdam-Rehbrücke, the Paul Langerhans Institute Dresden of the Helmholtz Zentrum München at the University Medical Center Carl Gustav Carus of the TU Dresden and the Institute for Diabetes Research and Metabolic Diseases of the Helmholtz Zentrum München at the Eberhard-Karls-University of Tuebingen together with associated partners at the Universities in Heidelberg, Cologne, Leipzig, Lübeck and Munich.
Scientific contact for media:
Dr. Ünal Coskun,
Paul Langerhans Institut Dresden des Helmholtz Zentrums München am Universitätsklinikum
und der Medizinischen Fakultät Carl Gustav Carus der TU Dresden
Membrane Biochemistry
Fetscherstrasse 74
01307 Dresden
E-Mail: uenal.coskun(at)tu-dresden.de
Press contact

Birgit Niesing
niesing(at)dzd-ev.de
+49 (0)89 3187-3971